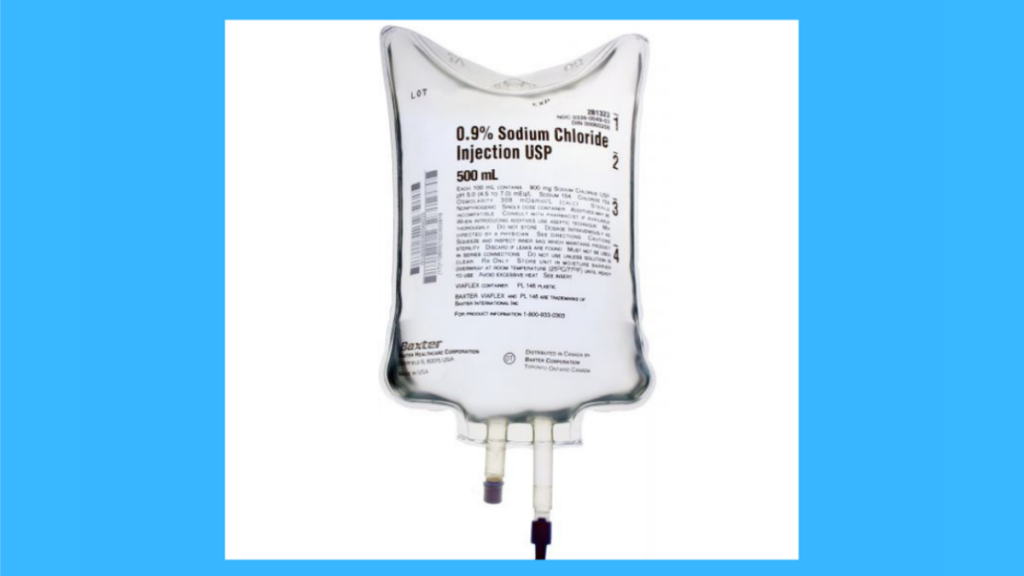
Sodium Chloride 0.9% IV Injection is a sterile solution of water for injection and sodium chloride intended for use as a solvent in testing blood, urine, and cerebrospinal fluid for glucose, ketones, and reducing substances and to provide the chloride ions necessary to prevent the formation of calcium phosphate precipitates. It is also used as an electrolyte replenisher, a hematinic solvent, in the reconstitution of parenteral solutions with dextrose, in fluid therapy before medical procedures, as an osmotic diuretic when administered peritoneally, and in other extracorporeal circulation techniques.
Sodium Chloride 0.9% IV Injection Common Uses
Sodium Chloride 0.9% IV Injection treats fluid and electrolyte imbalances. It is also used to help maintain blood pressure when fluids and electrolytes are lost due to vomiting, diarrhea, or sweating. This may prevent or treat dehydration caused by diarrhea, vomiting, or loss of body fluids in newborns and children.
This medication is given by injection into a vein, into a muscle, or under the skin. If you are using this medication at home, learn all preparation and usage instructions from your health care professional. Use exactly as directed. Do not use more of this product than prescribed without checking with your doctor first because doing so could cause harm.
Sodium Chloride 0.9% IV Injection Side Effects
Nausea, vomiting, and stomach pain. These can happen with any type of injection into the skin or muscle, including shots to treat allergies (immunotherapy). They’re most likely to happen within a few hours of the shot.
Pain at the injection site — this can be mild to severe. It’s normal to feel some soreness, but if the pain is severe or doesn’t go away after 1 to 2 days, call your doctor because you could have an infection.
Sodium Chloride 0.9% IV Injection Precautions
Sodium Chloride 0.9% IV Injection is a sterile solution of sodium chloride in water intended for intravenous infusion. It may be used as a fluid and electrolyte replacement source to treat dehydration due to diarrhea or gastrointestinal disturbances.
The solution contains no antimicrobial agents and should be used within 6 hours after reconstitution.
Sodium Chloride 0.9% IV Injection is supplied in single-dose containers containing 250 mL (8 fl oz) or 500 mL (16 fl oz). Each milliliter (mL) contains 90 mg sodium ion, 9 grams chloride ion, 16 mEq sodium ion, and 1 mEq potassium ion; pH is 4.5 to 6.5 at 20°C (68°F).
Sodium Chloride 0.9% IV Injection is contraindicated in patients with hypernatremia, hyperosmolar coma, or conditions predisposing to hyperosmolar coma such as myxedema or severe hypothyroidism; severe metabolic disorders such as ketosis; hyperchloremic acidosis; and conditions where fluid restriction is required such as congestive heart failure or cirrhosis with ascites formation.
Incompatibilities: Do not mix with other solutions or medications except those mentioned in this labeling.
Use sodium chloride 0.9% injection cautiously in:
- Patients with heart failure because sodium chloride 0.9% injection can cause fluid retention.
- Patients with diabetes mellitus because sodium chloride 0.9% injection may alter blood glucose levels.
- Patients with renal impairment, the risk of adverse events are increased (see Drug Interactions).
- Patients with hyperkalemia or hypokalemia (abnormal potassium levels) because sodium chloride 0.9% injection may worsen these conditions by increasing or decreasing serum potassium levels, respectively.
The Present Sodium Chloride Supply Chain Issues
Currently, the FDA is very well aware that the United States is experiencing supply chain disruptions with ongoing prefilled 0.9% sodium chloride shortages affecting healthcare everywhere.
The popular prefilled 0.9% sodium chloride IV lock/ flush syringes are in shortage because of the big increase in demand presented during the COVID-19 public health emergency, including the permanent discontinuance of certain prefilled saline lock/ flush syringes, as well as recent global vendor medical supply chain challenges too.